Introduction
The global biopharmaceutical landscape has undergone a profound transformation over the past few decades, driven largely by advances in recombinant DNA technology. Among the most impactful outcomes of this progress is the development of recombinant hormone biosimilars - biologic medicines designed to closely replicate already approved recombinant hormone therapies. These products are reshaping access to treatment for millions of patients worldwide by combining scientific rigor, clinical efficacy, and economic sustainability.
Definition
Recombinant hormone biosimilars are biologic medicines developed to be highly similar to an already approved recombinant hormone product, produced using recombinant DNA technology in living cells. They match the reference hormone in terms of structure, biological activity, safety, and clinical effectiveness, with no clinically meaningful differences, while often offering more affordable treatment options once patent protection of the original product expires.
Understanding Recombinant Hormones
Recombinant hormones are therapeutic proteins produced using genetic engineering techniques. Instead of being extracted from human or animal tissues - a practice once common but fraught with safety and supply concerns - these hormones are manufactured by inserting the human gene encoding the hormone into a host organism, such as bacteria, yeast, or mammalian cells. The host cells then express the hormone, which is purified and formulated into a medicine.
Common recombinant hormones include:
Insulin for diabetes management
Human growth hormone (hGH) for growth disorders
Erythropoietin (EPO) for anemia
Follicle-stimulating hormone (FSH) and luteinizing hormone (LH) for fertility treatments
Parathyroid hormone (PTH) for osteoporosis
These therapies have revolutionized care by offering high purity, consistent quality, and reduced risk of contamination.
What Are Biosimilars?
A biosimilar is a biologic medicine that is highly similar to an already approved reference biologic product, with no clinically meaningful differences in terms of safety, purity, and potency. Unlike small-molecule generics, biosimilars cannot be exact copies due to the inherent complexity of biologics and their manufacturing processes.
Recombinant hormone biosimilars, therefore, are not simple replicas but carefully engineered products that match the reference hormone as closely as current science allows. Minor differences in structure may exist, but these must be shown - through extensive analytical and clinical testing - to have no impact on therapeutic performance.
Scientific and Manufacturing Principles
The development of recombinant hormone biosimilars is a highly sophisticated process. It begins with reverse engineering the reference product to understand its molecular structure, post-translational modifications, and biological activity.
Key stages include:
Cell Line Development:
Scientists select and engineer a suitable host cell line capable of producing the hormone with the desired characteristics.Upstream Processing:
The engineered cells are cultured under tightly controlled conditions to maximize yield and consistency.Downstream Processing:
Purification steps remove impurities, host-cell proteins, and contaminants while preserving the hormone’s structure and activity.Analytical Characterization:
Advanced techniques such as mass spectrometry, chromatography, and bioassays are used to compare the biosimilar with the reference product at a molecular and functional level.Formulation and Stability Testing:
The final product is formulated for optimal stability, shelf life, and patient usability.
Consistency and reproducibility are critical, as even small changes in manufacturing conditions can affect the final biologic product.
Regulatory Pathways and Approval
Regulatory agencies around the world have established stringent frameworks to ensure the safety and efficacy of biosimilars.
FDA (United States): Approves biosimilars under the Biologics Price Competition and Innovation Act (BPCIA).
EMA (European Union): A global pioneer in biosimilar regulation, with detailed product-class-specific guidelines.
WHO: Provides international standards to support harmonization, particularly in emerging markets.
Approval is based on a totality of evidence approach, which includes:
Comprehensive analytical comparability
Non-clinical studies (if needed)
Clinical pharmacokinetic and pharmacodynamic studies
At least one confirmatory clinical trial in a sensitive indication
For recombinant hormone biosimilars, regulators may allow reduced clinical trial requirements when analytical similarity is convincingly demonstrated, helping accelerate access while maintaining safety.
Clinical Applications of Recombinant Hormone Biosimilars
Recombinant hormone biosimilars are now used across a wide range of therapeutic areas:
1. Diabetes Care
Insulin biosimilars have significantly expanded treatment options for patients with type 1 and type 2 diabetes. By lowering costs, they improve adherence and reduce the economic burden on healthcare systems.
2. Endocrine and Growth Disorders
Biosimilar human growth hormone is prescribed for pediatric growth hormone deficiency, Turner syndrome, and adult growth hormone deficiency, offering comparable outcomes at reduced cost.
3. Anemia Management
Erythropoietin biosimilars are widely used in patients with chronic kidney disease or chemotherapy-induced anemia, with extensive real-world evidence supporting their effectiveness.
4. Reproductive Medicine
Biosimilar gonadotropins play a crucial role in assisted reproductive technologies, making fertility treatments more accessible.
Benefits of Recombinant Hormone Biosimilars
Improved Affordability:
One of the most significant advantages is cost reduction. Biosimilars typically enter the market at lower prices than reference products, increasing competition and driving down overall healthcare spending.
Expanded Patient Access:
Lower costs enable more patients to receive life-saving or life-enhancing hormone therapies, especially in low- and middle-income countries.
Healthcare System Sustainability:
By reducing expenditure on biologics, biosimilars free up resources that can be reinvested in innovation, preventive care, and advanced therapies.
Proven Safety and Efficacy:
Years of post-marketing surveillance and real-world data have consistently shown that approved biosimilars perform as well as their reference products.
Challenges and Misconceptions
Despite their benefits, recombinant hormone biosimilars face several challenges:
Physician and Patient Awareness: Misunderstandings about biosimilar safety and interchangeability can slow adoption.
Manufacturing Complexity: High technical barriers require substantial investment and expertise.
Regulatory and Legal Hurdles: Patent litigation and market exclusivity periods can delay market entry.
Pharmacovigilance Requirements: Ongoing monitoring is essential to ensure long-term safety and maintain confidence.
Educational initiatives and transparent communication are essential to overcome these barriers.
Interchangeability and Switching
In some regions, certain biosimilars may be designated as interchangeable, meaning they can be substituted for the reference product without prescriber intervention. Achieving this status requires additional evidence demonstrating that switching does not increase risks or reduce efficacy.
For recombinant hormone biosimilars - particularly insulin - interchangeability has major implications for pharmacy practice, patient convenience, and healthcare costs.
The Future of Recombinant Hormone Biosimilars
The future outlook for recombinant hormone biosimilars is highly promising. Continued advancements in cell line engineering, analytics, and manufacturing automation are expected to further enhance product quality and reduce costs.
Emerging trends include:
Next-generation biosimilars with improved delivery devices
Global expansion into emerging healthcare markets
Digital health integration for monitoring and adherence
Greater harmonization of international regulatory standards
As patents for more recombinant hormone products expire, the biosimilar pipeline will continue to grow, fostering innovation and competition.
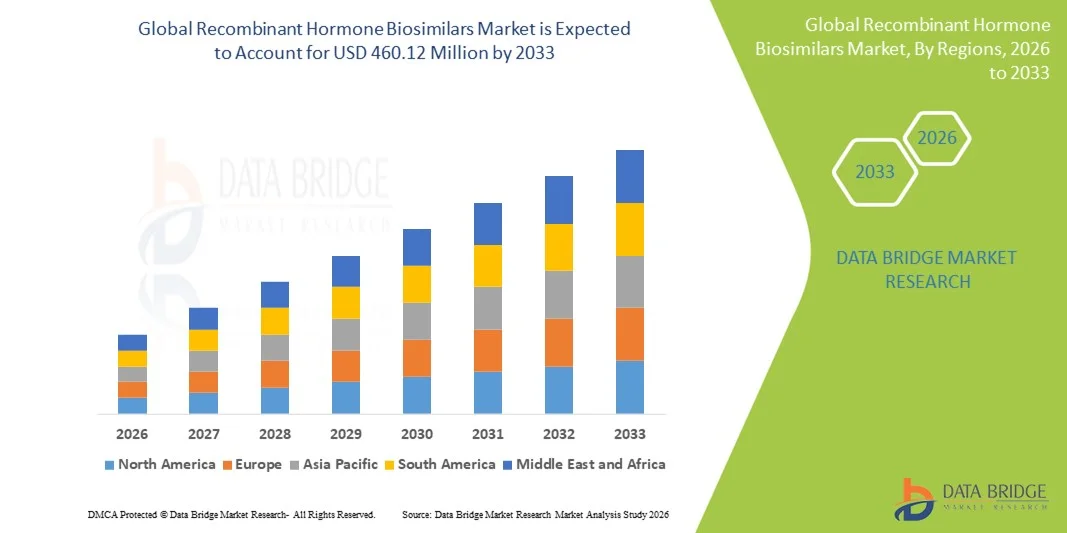
Growth Rate of Recombinant Hormone Biosimilars Market
According to Data Bridge Market Research, the recombinant hormone biosimilars market was estimated to be worth USD 217.80 million in 2025 and is projected to grow at a compound annual growth rate (CAGR) of 9.80% to reach USD 460.12 million by 2033.
Learn More: https://www.databridgemarketresearch.com/reports/global-recombinant-hormone-biosimilars-market
Conclusion
Recombinant hormone biosimilars represent a critical intersection of biotechnology, medicine, and health economics. By delivering therapies that match the safety and efficacy of established recombinant hormones at a lower cost, they are transforming patient care and strengthening healthcare systems worldwide.
Powered by Froala Editor
You may also like
More from this category.

The Power of Tandoor Ovens in Modern Commercial Kitchens
Tooth Extraction in West Delhi – Expert Oral Surgery Care | DentoHub

Vinny Pizza: A Slice of Authentic Flavor with a Modern Twist

County Pizza: Where Local Flavor Meets Legendary Taste
NextGen Diagnostic Imaging

Get the Perfect Smile with the Best Orthodontist in Langar House at FMS Dental

Best Dentist in Hyderabad – Patient-Focused Care at FMS Dental

Why Selenium Is the Most Popular Tool for Web Automation?
Anti-Aging Treatments: Modern Solutions for Youthful, Healthy-Looking Skin

