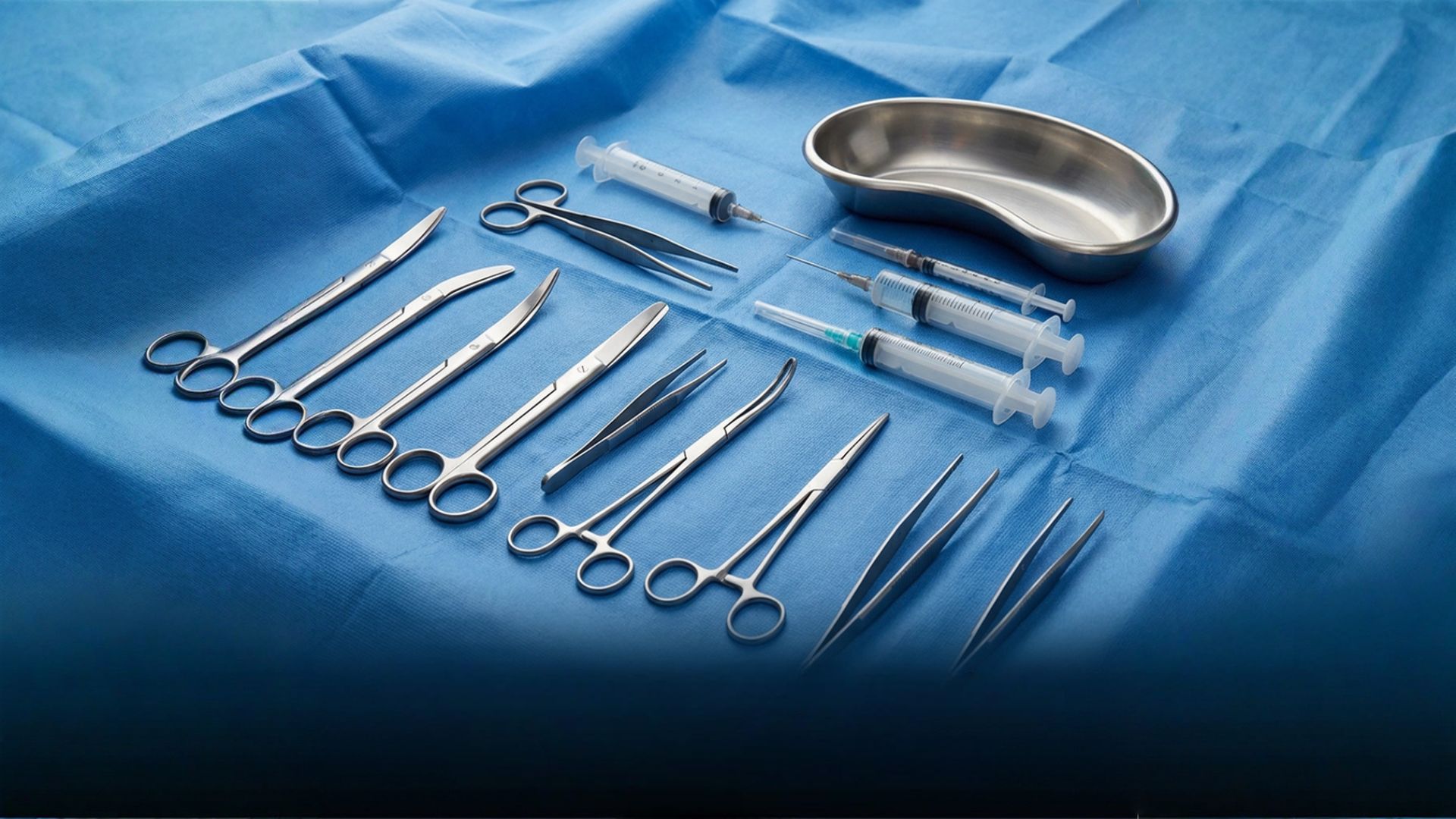
Why Surgical Instrument Manufacturers Can’t Afford to Ignore It
Ever held a surgical instrument in your hand and wondered, even for a fleeting second, how many checks it went through before it ended up in the operating room? For manufacturers, that question isn’t just idle curiosity—it’s the heart of why ISO 13485 certification exists. It’s the kind of standard that’s less about paperwork and more about life-and-death precision.
ISO 13485 isn’t just another badge to hang on the wall. Honestly, it’s a language of trust—a mark that says, “We take safety seriously. We take quality seriously. And yes, patients can rely on us.” For surgical instrument manufacturers, where even a tiny flaw can ripple into serious consequences, that message is everything.
What Exactly Is ISO 13485?
Let’s keep it simple. ISO 13485 is a standard that defines what a medical device manufacturer’s Quality Management System (QMS) should look like. It’s not just about producing instruments that function; it’s about producing instruments consistently, reliably, and safely.
Think of it this way: imagine baking a cake. You can follow a recipe once and hope for the best. But if you have to bake it many times for different customers, you need exact instructions, quality checks, and contingency plans for every step. That’s what ISO 13485 does for surgical instruments—it’s your recipe for consistently high-quality medical devices.
Certification isn’t just a badge—it’s a promise that safety comes first, every single time.
Why Manufacturers Should Care (Spoiler: It’s Not Just About Compliance)
Some people think ISO 13485 is just red tape, a bureaucratic headache you’d rather avoid. But here’s the kicker—skipping it can cost more than it saves.
Imagine this: a batch of instruments is found faulty during a procedure. One instrument, one incident, and suddenly, you’re facing recalls, damage to reputation, and serious trust issues. No certificate can fully erase that, but having ISO 13485 in place drastically lowers the risk because your processes are designed to catch problems before they reach the operating room.
It also shows clients and distributors that you take quality seriously. Think of it as a credibility shortcut in a highly skeptical market. And let’s be honest, in surgery, trust is everything.
The Nuts and Bolts of ISO 13485
Now, before your eyes glaze over thinking “here come the technical details,” bear with me—it’s not just about forms. The standard covers everything from document control to supplier management, all wrapped in a framework that encourages consistency. Here’s a breakdown, plain and simple:
Quality Management System (QMS): Your playbook. All procedures, responsibilities, and processes are documented. If something goes wrong, you know where to look.
Documentation & Records: Think SOPs, batch records, calibration logs. Yes, it’s a lot of paperwork, but it’s also the safety net that keeps your products reliable.
Risk Management: Not just a checkbox. You identify potential risks for each instrument, from material defects to user errors, and plan mitigation strategies.
Design & Development Controls: Each new instrument isn’t just made; it’s carefully designed, tested, and reviewed at multiple stages. Imagine building a bridge without engineering checks—you wouldn’t do it, right? Same with surgical tools.
Supplier & Process Controls: Your suppliers need to follow consistent quality practices. Raw materials, outsourced services, every link in the chain affects the end product.
It might sound rigid, but in practice, it allows for creativity within a safe boundary. You can innovate—but predictably. That balance is exactly what keeps surgeons and patients confident in your instruments.
Getting Certified: The Roadmap
If you think ISO 13485 certification is a one-day affair, let me stop you right there. It’s a journey, but a manageable one if you pace yourself.
Gap Analysis: Check your current processes against ISO 13485 requirements. This is where you discover the gaps. Some might be minor tweaks, others might require bigger shifts.
Internal Audit: Think of this as rehearsing before the big performance. It’s your chance to catch errors before an external review.
Corrective Actions: Fix what’s broken, streamline what’s messy, and document it. This step is tedious but crucial—nothing impresses a reviewer like clean, organized records.
Certification Audit: An external reviewer examines your QMS. If everything checks out, congratulations! You’re officially ISO 13485 certified.
You know what? Many manufacturers find the process surprisingly eye-opening. It forces you to look under every rock, check every bolt, and truly understand your production process. And the payoff isn’t just a certificate—it’s a system that genuinely improves quality.
Common Challenges—and How to Survive Them
Every journey has bumps. ISO 13485 certification is no exception. But knowing what’s coming helps you navigate it.
Paperwork Overload: Yes, there’s a mountain of documentation. Solution? Use digital platforms to automate and organize much of it.
Resistance to Change: Staff might grumble, especially those used to “the old ways.” Answer? Training, patience, and showing how these changes actually make their work easier in the long run.
Supplier Alignment: Your suppliers are only as good as their processes. Work with them early, share your expectations, and make reviews a partnership, not a punishment.
Here’s the truth—sometimes it feels like you’re swimming against the current. But once you reach the shore, the clarity and control you gain over your operations are worth it.
Tools and Resources That Make Life Easier
No need to reinvent the wheel. Several resources make ISO 13485 certification manageable:
Digital QMS Software: Helps automate documentation, track compliance, and simplify audits.
Templates and Checklists: Pre-made templates can save countless hours, especially for SOPs and risk assessments.
Training Providers: Workshops or online courses ensure your team understands both the “how” and the “why.”
Honestly, using the right tools can make certification feel less like a chore and more like building a robust foundation for growth.
The Payoff: Beyond the Certificate
Let’s not sugarcoat it—ISO 13485 isn’t a magic wand. It doesn’t instantly make your instruments flawless. But here’s the thing: it systematically reduces mistakes, strengthens your processes, and boosts confidence across the board.
For manufacturers of surgical instruments, that translates to real, tangible benefits:
Safer Instruments: Fewer defects, better design controls, and consistent quality.
Market Access: Certification is recognized broadly, so expanding your reach becomes easier.
Reputation and Trust: Surgeons and patients alike trust your brand more when your instruments carry a mark of certified quality.
And at a human level, there’s a quiet pride in knowing the tools you make might be part of life-saving procedures—made to the highest standard possible. That’s worth more than a certificate on the wall.
Closing Thoughts
So, could your instruments withstand a rigorous ISO 13485 review today? If the answer isn’t a confident “yes,” it might be time to start.
ISO 13485 isn’t just bureaucracy—it’s a framework that protects patients, supports manufacturers, and elevates the standards of surgical care everywhere. For surgical instrument manufacturers, certification is more than a process; it’s a promise—a commitment to quality, safety, and trust that goes far beyond the production line.
In high-stakes surgery, it’s the difference between instruments you hope are safe and instruments you know are safe. And honestly, in healthcare, that’s a difference worth certifying.
Powered by Froala Editor
You may also like
More from this category.

The Power of Tandoor Ovens in Modern Commercial Kitchens
Tooth Extraction in West Delhi – Expert Oral Surgery Care | DentoHub

Vinny Pizza: A Slice of Authentic Flavor with a Modern Twist

County Pizza: Where Local Flavor Meets Legendary Taste
NextGen Diagnostic Imaging

Get the Perfect Smile with the Best Orthodontist in Langar House at FMS Dental

Best Dentist in Hyderabad – Patient-Focused Care at FMS Dental

Why Selenium Is the Most Popular Tool for Web Automation?
Anti-Aging Treatments: Modern Solutions for Youthful, Healthy-Looking Skin

